Analytical Similarity Assessment in Biosimilar Product Development
by Shein-Chung Chow
2020-07-24 03:52:52
Analytical Similarity Assessment in Biosimilar Product Development
by Shein-Chung Chow
2020-07-24 03:52:52
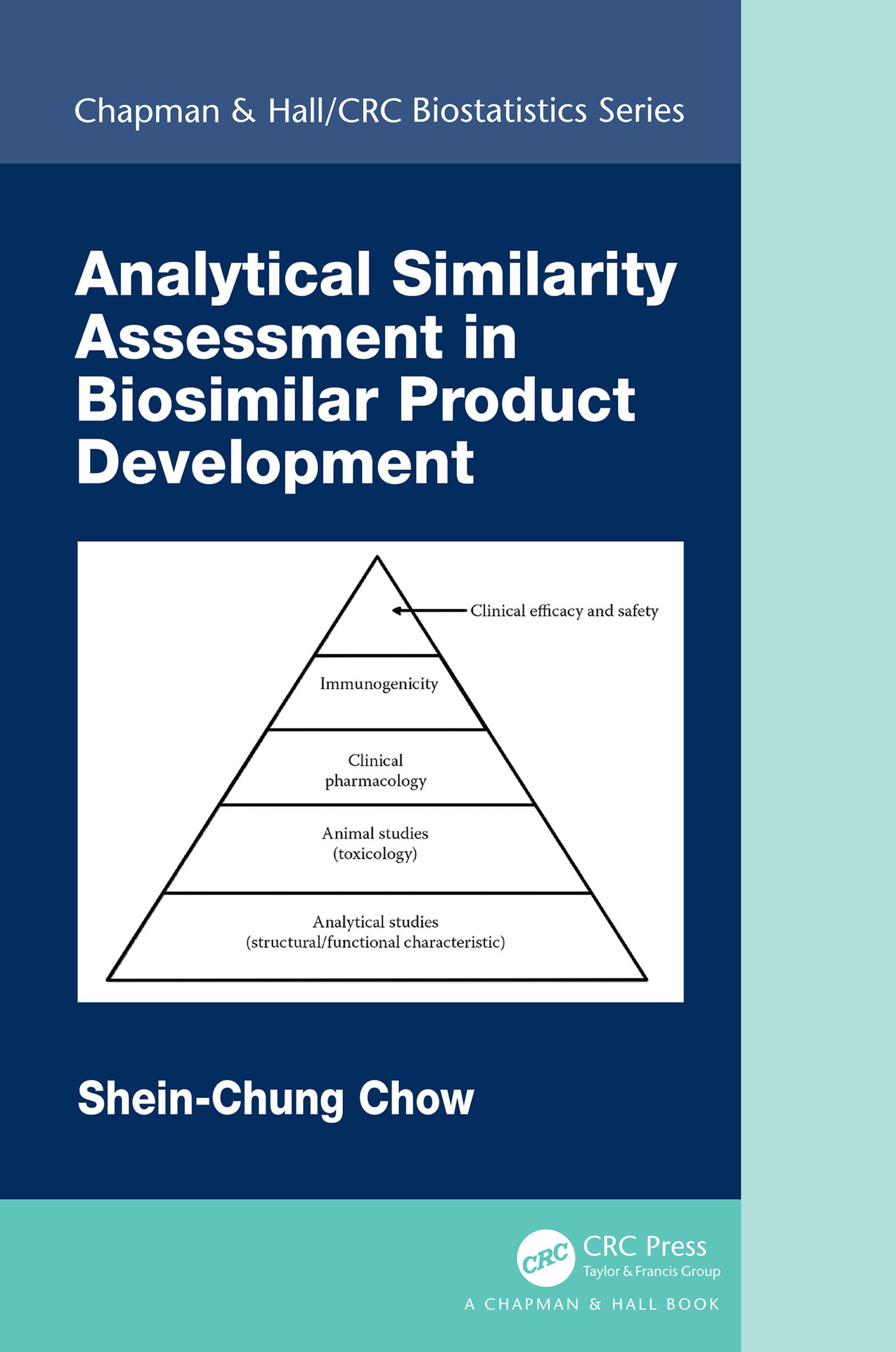
This book focuses on analytical similarity assessment in biosimilar product development following the FDAâs recommended stepwise approach for obtaining totality-of-the-evidence for approval of biosimilar products. It covers concepts such as the ...
Read more
This book focuses on analytical similarity assessment in biosimilar product development following the FDAâs recommended stepwise approach for obtaining totality-of-the-evidence for approval of biosimilar products. It covers concepts such as the tiered approach for assessment of similarity of critical quality attributes in the manufacturing process of biosimilar products, models/methods like the statistical model for classification of critical quality attributes, equivalence tests for critical quality attributes in Tier 1 and the corresponding sample size requirements, current issues, and recent developments in analytical similarity assessment.
Less